A Glimpse into the Single-Use vs. Reuse Medical Device Market in EU
Brought to you by Long Tran, Nazokatkhon Akhmadjonova, Pilar Gonzalez Guevara, Camille Rønn and Daniel Fürstenau
What does Europe’s medical device database reveal about the dominance of single-use products, and the opportunities for reuse?
Single-use medical devices account for a large share of healthcare’s environmental footprint. A recent study estimated that the production, transportation, use, and disposal of single-use products contribute up to 80% of the sector’s total carbon emissions. Despite growing commitments from organizations such as MedTech Europe, the NHS in the UK, and the European Commission’s Green Deal, the transition toward reusable products and circular medical technologies remains slow.
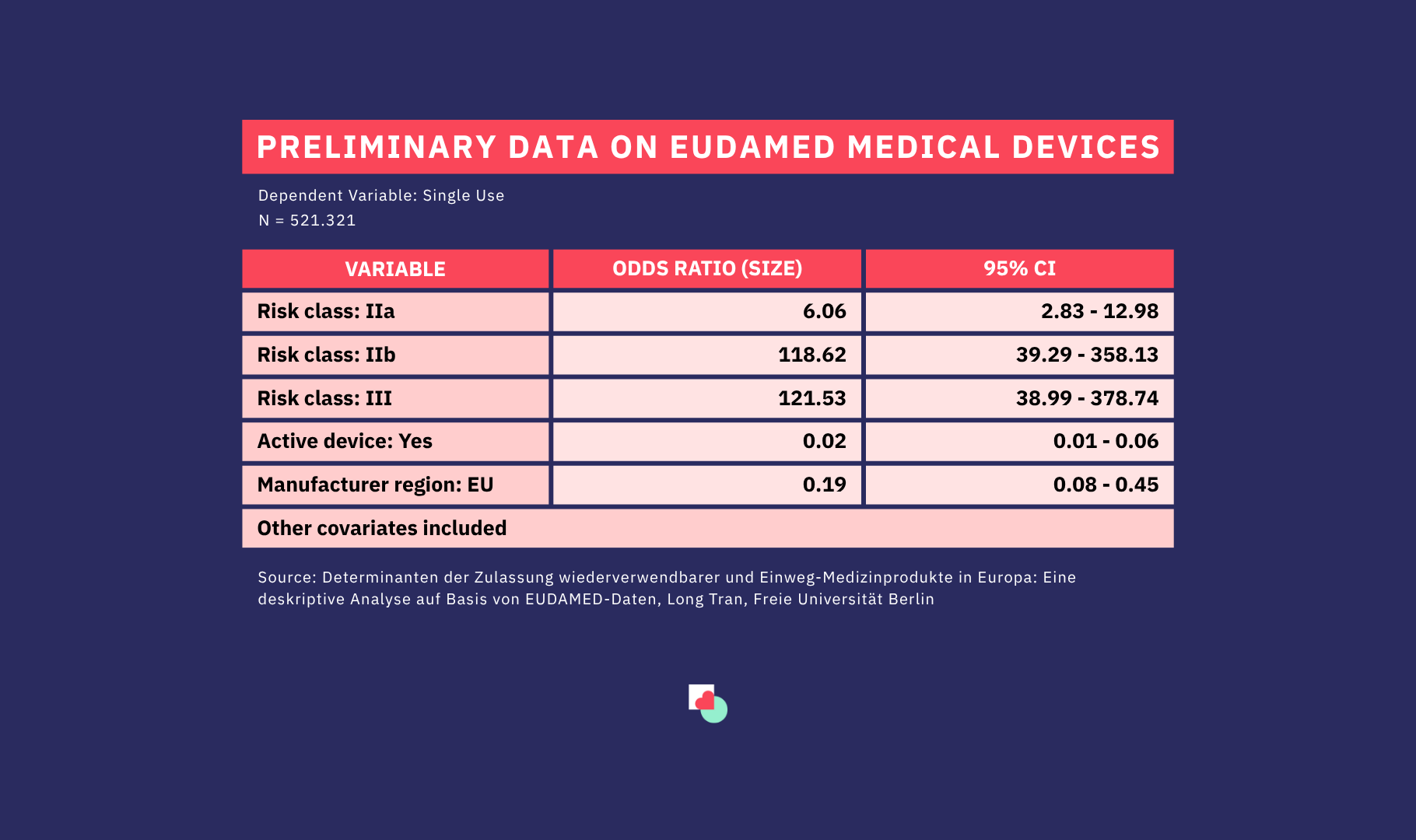
One place where this transformation can be observed is the EUDAMED database, a central EU platform where medical devices approved for the European market are registered. Drawing on an analysis of 526,826 unique product listings, a recent master’s thesis by Long Tran (FU Berlin) explores the characteristics that determine whether a device is labelled as single-use or reusable. Using logistic regression models, the study examines how product type, manufacturer origin, and regulatory classification shape reuse practices across the industry. Below are a few preliminary facts:
Fact 1: Products from European manufacturers are significantly less likely to be single use compared to non-European manufacturers when selling their products in EU
According to the thesis’ findings, devices from EU manufacturers are 81% (or more than 5 times) less likely to be labelled single use compared to those who originate outside the EU, corresponding to an odds ratio (OR) of 0.19. This could reflect the European market’s stronger emphasis on environmental sustainability and waste reduction or perhaps that hospitals in Europe perceive the use of reusable devices as less risky in terms of contamination compared to single-use products.
Fact 2: Risk class is the single strongest determinant of single-use status vs. reusable
The analysis shows that devices in higher MDR risk classes, especially Risk Class IIb and III, are overwhelmingly likely to be single use. Risk Class III devices are approximately 121 times more likely to be registered as single use compared with Risk Class I devices. This strong relationship highlights the role of regulation and patient safety concerns when products come in contact with human tissue and blood. So, with high-risk products, single use is the default.
Products under the MDR framework are less often single use compared to those registered under older directives (such as MDD), pointing to evolving types of products, regulation and perhaps environmental expectations.
Fact 3: Active devices are strongly associated with reuse
Active devices, those incorporating electronic or powered components, made up roughly 8% of all items in the dataset. Active devices show odds ratios around 0.01–0.02, indicating they are overwhelmingly registered as reusable, meaning they are 98% (or 50 times) less likely to be single-use than non-active devices. This aligns with the nature of such devices: they tend to be of higher value, with complex technologies for which reuse is economically and technically beneficial.
Insights and outlook
Understanding the structures behind the single-use market is essential for deciding where future efforts toward circularity should focus. These are some of the first preliminary descriptive insights from a study that will be conducted on single use vs. reusable devices on the EU market. Stay tuned, for more exciting research!